Catalysts
A catalyst is a substance that increases the rate of a chemical reaction without itself undergoing any permanent chemical change.
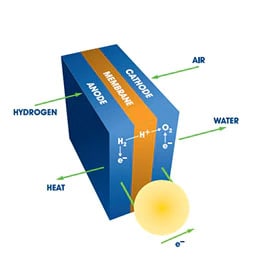
Chemical reactions occur faster in the presence of a catalyst because the catalyst provides an alternative reaction pathway with a lower activation energy compared to non-catalyzed reactions. The catalyst is not consumed in the process and can continue to act repeatedly, therefore only very small amounts of catalyst are required to alter the rate of a chemical reaction. We offer a full range of metal catalysts in varying purities and concentrations that includes homogeneous catalysts, supported/unsupported heterogeneous catalysts and fuel cell catalysts for anodes, cathodes, electrodes.
Featured Products

Precious metal compounds (homogeneous)